南杰企讯|药欣生物与INSIGNIS THERAPEUTICS公司联合开发的过敏反应新药获美国FDA批准开展临床试验
收藏
关键词:
临床联合开发新药FDA批准FDA生物药
资讯来源:汉口路22号生物医药 + 订阅账号
所属行业:化学药制剂 + 订阅行业
发布时间:
2020-11-04
药欣生物和INSIGNIS THERAPEUTICS公司联合开发用于治疗过敏反应的肾上腺素前药舌下口崩片(药欣生物项目代码HLK-0006,Insignis 项目代码IN-001)新药获美国FDA批准开展临床试验。
美国康州消息,2020年10月29日,深圳市药欣生物科技有限公司(以下简称“药欣生物”)与美国Insignis Therapeutics公司(以下简称“Insignis”)联合研发的HLK-0006创新制剂获得美国FDA批准开展临床试验,并将于2020年12月在美国开展I期临床试验研究。此次临床试验的主要目的是在健康人体内进行HLK-0006舌下口崩片的剂量探索和安全性验证,预计将在2021年初获得临床数据。届时,药欣生物将继续携手Insignis于2021年下半年启动关键性临床研究,未来将以505(b)(2)申报途径在美国申请NDA。同时药欣生物将大力推进HLK-0006舌下口崩片在国内的开发和上市。
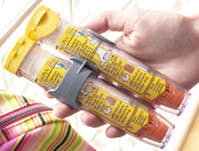
HLK-0006是一种肾上腺素前药舌下口崩片制剂(图1),用于治疗危及生命的过敏性休克。作为一种速效舌下口崩片,服用时不需要水或吞咽,在任何时间任何地点任何人均可快速使用,相比目前的肾上腺素注射笔(图2),在患者依从性方面、稳定性方面等具备显著的优点。
药欣生物CEO兼联合创始人徐俊女士称:“作为战略合作人,我们对HLK-0006获得FDA批准开展临床试验感到非常高兴。我们相信HLK-0006有希望成为过敏性休克的一个变革性治疗药物,因为它作为一种舌下口崩片制剂,更容易被患者接受,患者无需再使用注射产品。”
Insignis创始人兼CEO张明宝博士表示:“全球肾上腺素市场价值数十亿美元,随着过敏反应流行率和发病率的增加,这一市场还在继续增长。我们相信,IN-001将会为患者提供一种更方便和快速的治疗方案,以取代目前紧急情况下使用的注射产品。FDA批准对IN-001舌下口崩片的 IND申请是Insignis和药欣生物的一个重要里程碑,因为我们共同推进了IN-001的开发,为严重过敏患者提供了一种肾上腺素前药舌下口崩片制剂,以应对危及生命的过敏性休克等紧急情况的发作。”
Insignis 科学顾问兼美国西奈山医学院儿科教授Julie Wang博士表示:“肾上腺素为治疗过敏反应的首选药物。然而,随身携带自动注射笔的不便以及对针头的恐惧是及时使用这种救命药的主要障碍。一种可替代肾上腺素注射产品的患者友好型药物则可以减少治疗延迟,而这个舌下口崩片则极有潜力解决这一关键需求。”
药欣生物董事长及联合创始人王泽人博士称:“欧美有约3%的过敏患者,国内病人逐年增加,发病率也已接近欧美。我们希望HLK-0006产品可取代注射笔,满足超敏反应患者自救需求并填补国内外空白。开发best-in-therapeutics 产品是我们的宗旨。”
深圳市药欣生物科技有限公司是一家致力于创新药研发和产业化的生物医药公司,研发总部位于深圳市南山区高新科技园,在美国康州拥有全资子公司HLK Pharmacin。公司致力于开发改善患者预后的同类最佳疗效(best-in-therapeutics)药物,其自主研发的用于治疗乳腺癌新药HLK-1001胶囊已于2019年9月获得美国FDA同意临床试验,并完成pilot临床达到预期。从科学理念到商业化,药欣生物凭借世界领先的专业技术知识以及独特的制剂平台,助推高附加值药物快速上市,获取更高的成本效益。
更多信息请访问:
ht
tps://pharmacin.com/
;
https://hl
kpharma.com/
Insignis Therapeutics是美国一家专注于开发治疗过敏反应创新替代药物的生物技术公司,其重磅产品IN-001是一种肾上腺素前药的舌下口崩片,
它为患者提供了一种比现有肾上腺素注射产品更方便的选择,以应对危及生命的紧急情况。更多信息请访问http://www.insignisrx.com/。
NORTH HAVEN/NEW HAVEN, CONNECTICUT,October 29, 2020 (GLOBE NEWSWIRE) – Insignis Therapeutics (“Insignis”), a biotech company focused on developing innovative therapeutics for anaphylaxis treatment and HLK Pharmacin, a global drug development company, today announced the U.S. Food and Drug Administration (FDA) has cleared its Investigational New Drug (IND) application for IN-001, an oral formulation of an epinephrine prodrug for life-saving treatment of allergic reactions, including anaphylaxis. Under this IND, Insignis in collaboration with its strategic partner, HLK Pharmacin, is prepared to initiate a Phase 1 study with IN-001 (known as HLK-0006 within HLK Pharmacin) and intends to have clinical data in first half of 2021.
The Phase 1 single dose study in healthy volunteers will compare IN-001 orally dissolving tablet (epinephrine prodrug) vs the current standard of care, EPIPEN (epinephrine injection). If successful, under the 505(b)(2) regulatory pathway, Insignis and HLK Pharmacin would begin preparations to initiate a pivotal study in the second half of 2021.
“The FDA clearance of our IND represents an important milestone for Insignis and our strategic partner HLK Pharmacin as we advance the development of IN-001 to provide people living with severe allergies an oral prodrug version of epinephrine for life-threatening emergencies such as when anaphylaxis strikes,” commented Mingbao Zhang, PhD, Chief Executive Officer and Founder of Insignis Therapeutics. “The global anaphylaxis injectable epinephrine market is a multi-billion dollar opportunity that is continuing to grow with the increase in prevalence and incidence of anaphylaxis. We believe that IN-001 has the potential to provide patients with a more discreet, convenient and fastacting alternative to current emergency injectable treatment options.”
HLK Pharmacin CEO andCo-Founder Ms. Jun Xu commented, “As a strategic collaborator, we are verypleased with the FDA IND clearance. We believe IN-001 has the potential to be a transformative treatment for anaphylaxis since it is a patient compatible, oral formulation and eliminates the need for an injectable product.”
IN-001 is a fast acting, orally dissolving tablet formulation that doesn’t require water or swallowing, potentially allowing rapid administration of life-saving epinephrine by anyone,anywhere and anytime. As an ambient, temperature stable oral prodrug form of epinephrine, IN-001 provides benefits over the current injectable formulation including ease of use, accessibility and longer shelf life.
Julie Wang, MD,Professor of Pediatrics at Mont Sinai School of Medicine, and Scientific Advisor to Insignis Therapeutics, commented, “Epinephrine is the key treatment for anaphylaxis. However, the inconvenience of carrying autoinjectors and fear of needles are known barriers to timely administration of this life-saving medication. A patient-friendly alternative to injectable epinephrine can reduce treatment delays, and the IN-001 orally dissolving tablet has the potential to address this critical need.”
About Insignis Therapeutics
Insignis Therapeutics is a NorthHaven, CT based biotech company focused on developing innovative therapeutics for anaphylaxis treatment. Its lead drug candidate, IN-001, is an orally dissolving tablet of epinephrine prodrug that provides patients with a convenient oral option over currently available injectable epinephrine for life-threatening allergic emergencies. The Company has received US FDA clearance ofits IND application and is preparing to initiate a Phase 1 study to evaluate the use of IN-001 for anaphylaxis. Formore information visit http://www.insignisrx.com/.
About HLK Pharmacin
HLK Pharmacin is a global drug development company based in New Haven, CT with corporate headquarters in Shenzhen,China. HLK Pharmacin is committed to improving patient outcomes through the development of best-in-class therapeutics. From concept through commercialization, HLK Pharmacin deploys state-of-the-art technology to enablevalue-added medicines to be realized faster and more cost effectively. For more information visit https://pharmacin.com/.
Company Contact:
Mingbao Zhang, PhD
203-570-8879
mzhang@insignisrx.com
内容仅供感兴趣的个人谨慎参考,非商用,非医用、非投资用。
版权归拥有者。欢迎朋友们指正!衷心感谢!
版权声明:文中图片取自网络,根据CC0协议使用,版权归拥有者。
任何问题,请与我们联系。衷心感谢!
药欣生物CEO兼联合创始人徐俊女士
同时担任南京大学生物医药行业校友会理事
南京大学生物医药行业校友会于2017年10月21日成立,是南京大学全球生物医药领域校友自愿结成的公益性、学习型校友组织。旨在服务生物医药领域的南京大学校友,为校友沟通交流、相互合作、创新创业提供分享平台。
校友会目前在全球拥有2000多名会员,包括8位院士,15位国家级人才计划专家,上百家创业企业家(超过8家上市公司),以及各大顶级高校从事生物医药领域的专家学者。
校友会设有秘书处,同时在全球多个城市设有联络人,方便活动的组织,同时聘请有行业内资深人士担任顾问,为校友活动提供指导。








 个人中心
个人中心
 我是园区
我是园区





