▎药明康德内容团队编辑
本周,多家大型医药公司开始公布2021年第一季度财报以及公司的最新进展。今天药明康德内容团队将与读者分享罗氏(Roche)和渤健(Biogen)公司的最新研发动向。罗氏公司面对新冠大流行,在开发新冠病毒的检测和疗法方面取得了显著进展。渤健公司已经向巴西、加拿大、瑞士和澳大利亚递交了其在研阿尔茨海默病(AD)抗体疗法的aducanumab的监管申请,与Sage Therapeutics公司合作开发治疗抑郁症和运动障碍的创新疗法也获得积极临床结果。
罗氏

罗氏在新冠大流行爆发以来,在新冠病毒诊断方面推出了数十种检测方法,在第一季度财报上的电话会议上,罗氏高管介绍了该公司开发的快速抗原检测,这款检测手段能够在15分钟内以99.1%的特异性检测出受到新冠病毒感染的患者。此外,该公司还开发了新冠突变病毒核酸检测,能够定性区分新冠病毒携带的E484K、N501Y、以及HV-69/70缺失变异。这将有助于追踪不同新冠突变病毒株在人群中的流行程度。
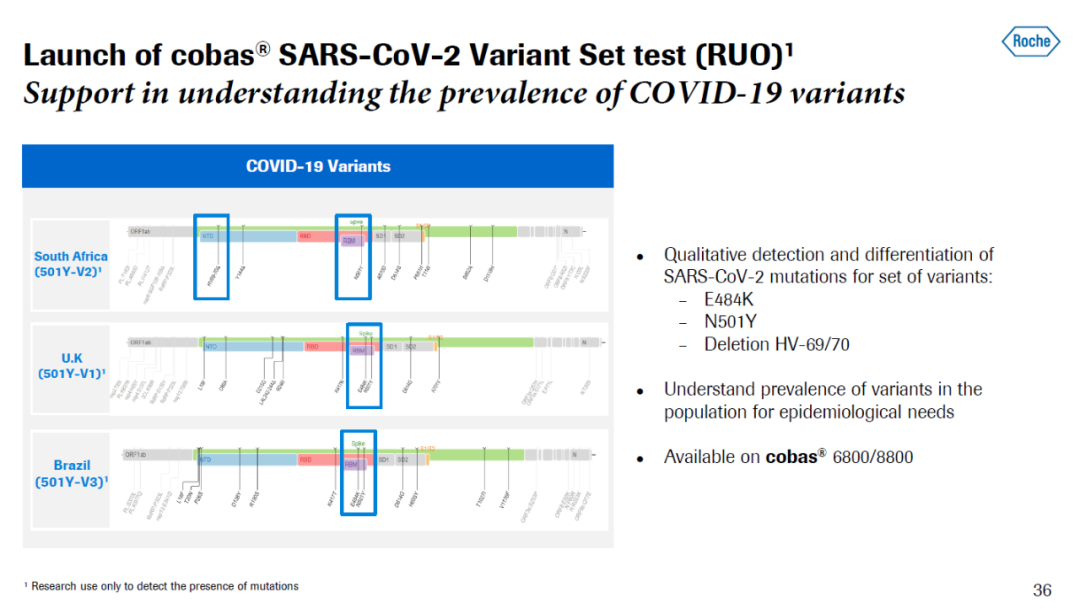
▲罗氏开发的新冠突变病毒检测能够帮助追踪突变病毒株在人口中的流行程度(图片来源:罗氏官网)
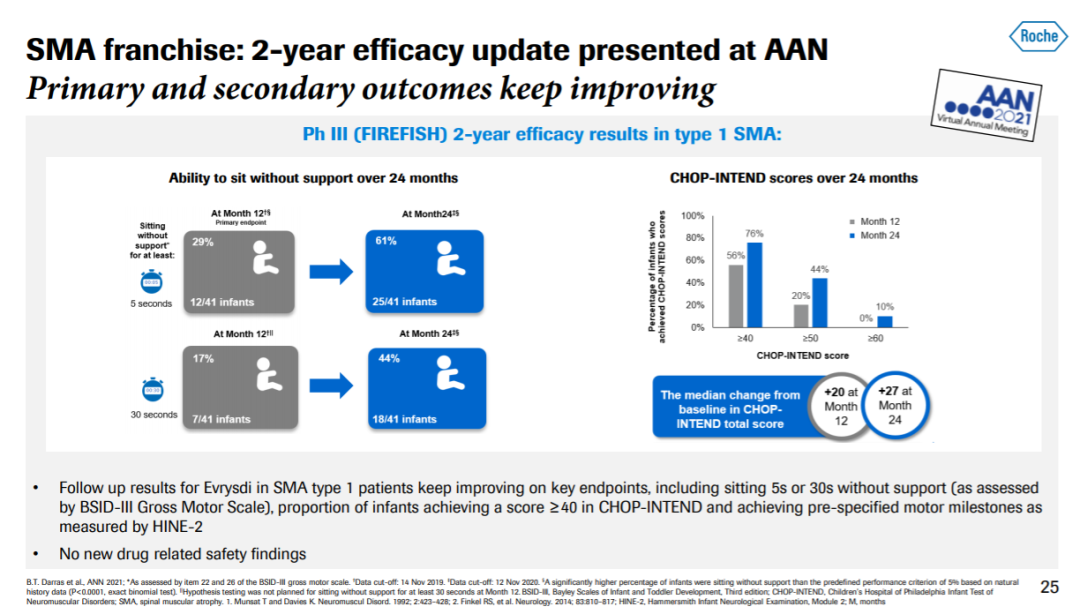
▲Evrysdi持续改善SMA患者症状(图片来源:罗氏官网)
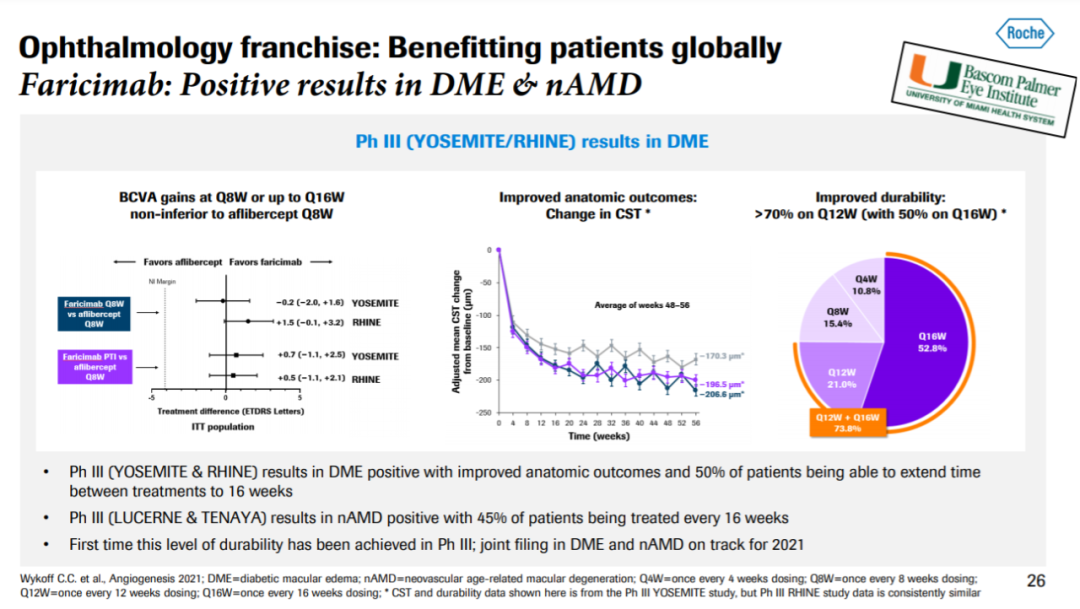
▲Faricimab在治疗nAMD和DME患者的3期临床试验中获得积极结果(图片来源:罗氏官网)
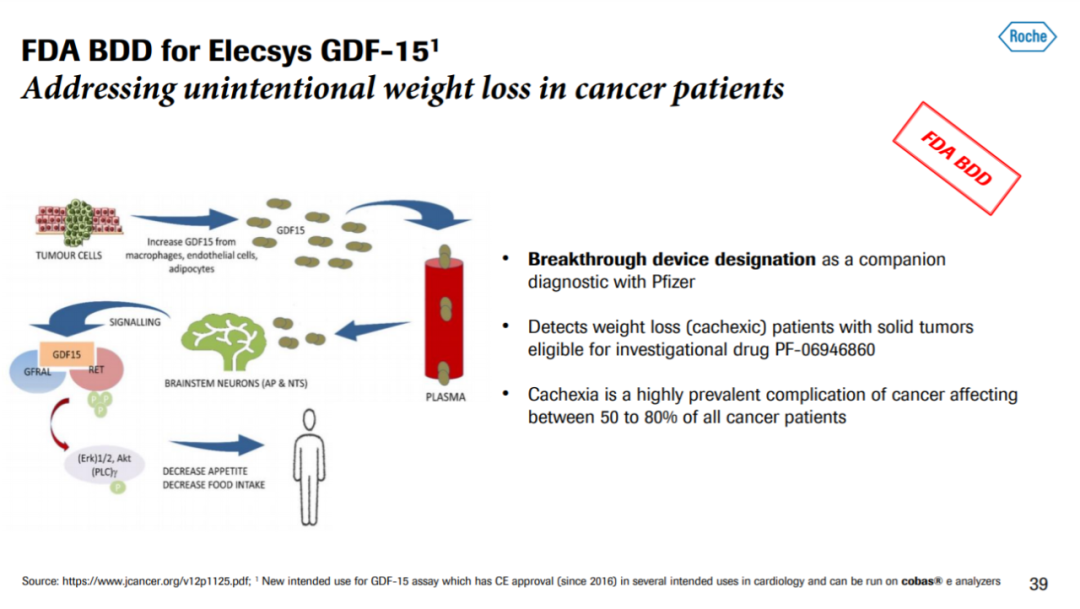
渤健


▲渤健在阿尔茨海默病研发方面的进展(图片来源:渤健官网)
渤健去年与Sage Therapeutics公司达成研发合作,共同开发用于治疗抑郁症的潜在“first-in-class”GABAA受体别构调节剂zuranolone和治疗原发性震颤的SAGE-324。Zuranolone近日在治疗重度抑郁症(MDD)患者的开放标签3期临床试验中获得积极结果。超过70%接受剂量为30 mg的zuranolone治疗的患者和80%接受剂量为50 mg的zuranolone治疗的患者在接受治疗15天时就获得积极应答。治疗原发性震颤的BIIB124(SAGE-324)在2期临床试验中也达到主要终点。
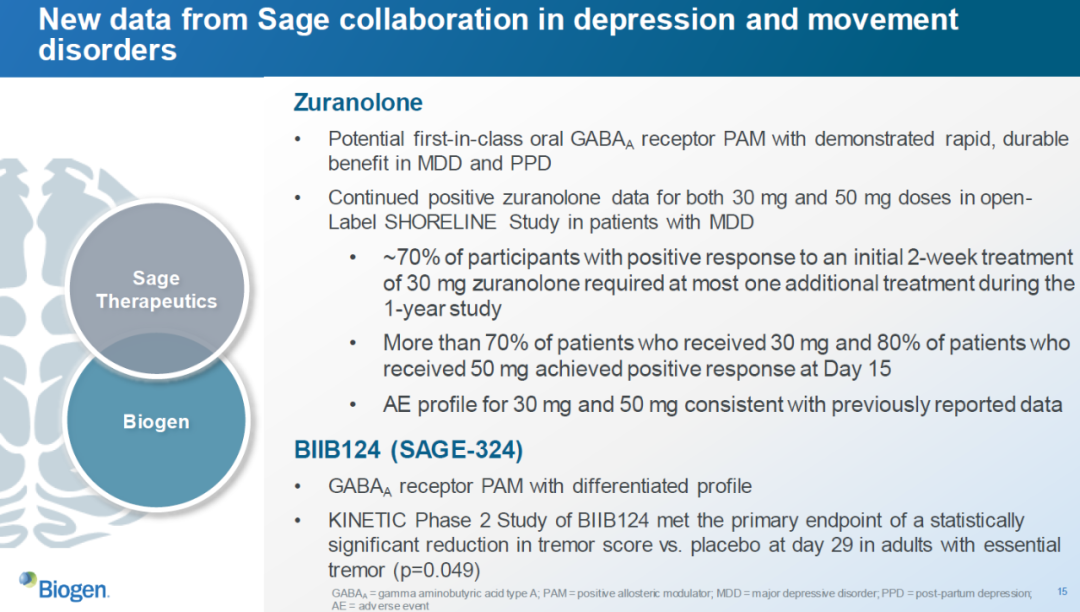
▲渤健和Sage合作开发的在研疗法在治疗抑郁症和运动障碍方面获得积极进展(图片来源:渤健官网)
参考资料:
[1] Roche reports solid results in the first quarter of 2021. Retrieved April22, 2021, from https://www.roche.com/media/releases/med-cor-2021-04-21.htm
[2] Roche Q1 2021 Sales. Retrieved April 22, 2021, from https://www.roche.com/dam/jcr:c5ff437f-ab27-4e0a-a479-d7a6bbf2b9ce/en/irp210421-a.pdf
[3] BIOGEN REPORTS FIRST QUARTER 2021 RESULTS. RetrievedApril 22, 2021, from https://investors.biogen.com/static-files/191cdae2-bb39-493e-9d4a-3a516e2e6281
[4] Biogen First Quarter 2021, financial results andbusiness update. Retrieved April 22, 2021, from https://investors.biogen.com/static-files/fe6f01a4-aec3-4bc0-90fa-221f96a4100f
[5] Roche’s Evrysdi continues to improve motor function andsurvival in babies with Type 1 Spinal Muscular Atrophy (SMA). Retrieved April22, 2021, from https://www.roche.com/media/releases/med-cor-2021-04-15.htm
[6] Phase III prevention trial showed subcutaneousadministration of investigational antibody cocktail casirivimab and imdevimabreduced risk of symptomatic COVID-19 infections by 81%. Retrieved April 22,2021, from https://www.roche.com/media/releases/med-cor-2021-04-12.htm
[7] New two-year data show Roche’s Evrysdi (risdiplam)continues to demonstrate improvement or maintenance of motor function in peopleaged 2-25 with Type 2 or Type 3 Spinal Muscular Atrophy (SMA). Retrieved April22, 2021, from https://www.roche.com/media/releases/med-cor-2021-03-16.htm
[8] New phase III data show Roche’s faricimab is the firstinvestigational injectable eye medicine to extend time between treatments up tofour months in two leading causes of vision loss, potentially reducingtreatment burden for patients. Retrieved April 22, 2021, from https://www.roche.com/media/releases/med-cor-2021-02-12.htm
[9] Sage Therapeutics Announces Continued PositiveZuranolone Data for Both 30 mg and 50 mg Doses in Open-Label SHORELINE Study inPatients with MDD. Retrieved April 22, 2021, from https://investor.sagerx.com/news-releases/news-release-details/sage-therapeutics-announces-continued-positive-zuranolone-data
版权说明:本文来自药明康德内容团队,欢迎个人转发至朋友圈,谢绝媒体或机构未经授权以任何形式转载至其他平台。转载授权请在「药明康德」微信公众号回复“转载”,获取转载须知。



 个人中心
个人中心
 我是园区
我是园区




