NEWS
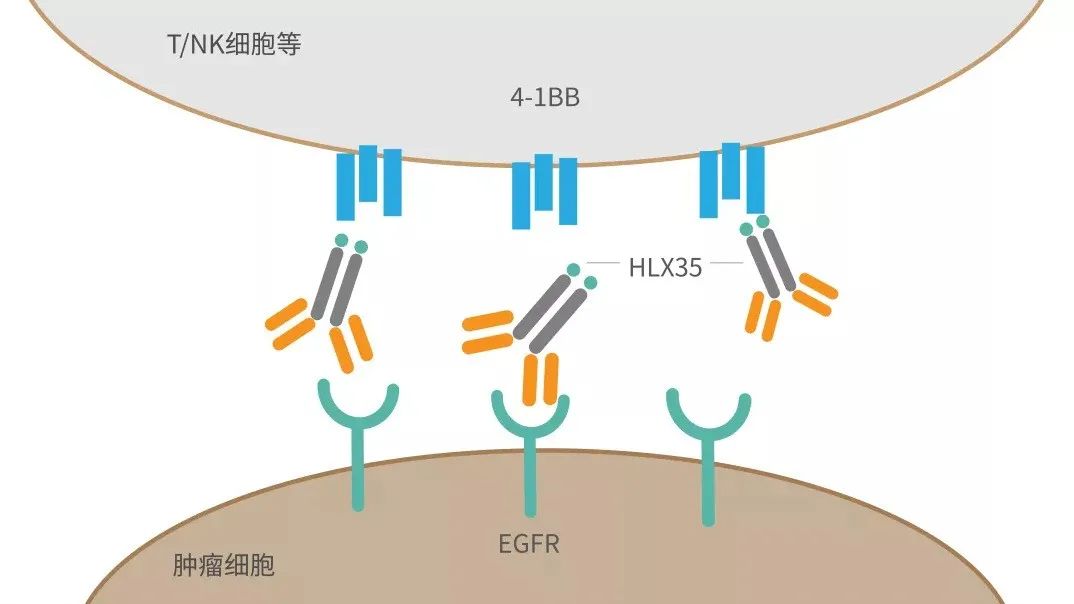
HLX35是公司自主研发的创新型抗EGFR和抗4-1BB双靶点的双特异性抗体。根据临床前研究结果,HLX35显示出比抗4-1BB或抗EGFR单抗的单一或联合治疗都更加优越的肿瘤抑制效果。双特异性抗体药物可有效将两个靶点的优势合并在一起,HLX35可以结合在肿瘤表面的EGFR分子上,阻断EGFR的激活和下游信号通路的磷酸化,杀死肿瘤细胞;同时还可以在EGFR的参与下,结合免疫细胞(T细胞和NK细胞)表面上的4-1BB免疫激活分子,使更多的免疫细胞聚集在肿瘤周围,并刺激微环境中免疫细胞的活性,从而协同杀死肿瘤细胞,提高疗效。2020年11月,公司就HLX35与Binacea pharma Inc. 达成独家许可协议,授予其于除中国(包括港澳台地区)以外的全球范围进行研究、开发、生产和商业化权利。
复宏汉霖从临床需求出发,目前已打造出多元化的创新产品管线,在PD-1/L1、CTLA-4、LAG-3等免疫检查点全面布局,为免疫联合治疗的探索创造更多可能。公司持续丰富创新靶点布局,产品覆盖c-MET、TROP2、TIGIT和BRAF等新兴靶点,并积极开展双特异性抗体、抗体偶联药物(ADC)等产品的开发,同时公司将持续加码创新,加强优质创新资产的引进和合作,“内外兼修”,为全球患者带去高质量、可负担的创新治疗方案。
关于复宏汉霖
会议推荐
2022第七届易贸生物产业大会EBC暨易贸生物产业展览
联合主办方

支持单位

官方合作伙伴

会议信息
大会名称:2022第七届易贸生物产业大会EBC暨易贸生物产业展览
主办单位:易贸医疗
指导单位:苏州工业园区管理委员会
协办单位:苏州工业园区投资促进委员会
会议地点:苏州国际博览中心
参与群体:覆盖创业者、科学家、临床医生、投资人和供应商等
易贸生物产业展览
EBC覆盖体外诊断、抗体药物、细胞治疗、基因治疗、mRNA等生物医药创新领域,通过EBC会议、展览、活动三种形式,共建生物产业交流合作平台。三大展馆全方位展示,B1展馆坐拥业内优秀品牌企业,更有大型娱乐展区,采访区,易贸快闪店;C1展馆集聚各类精彩品牌企业,更有约见区,观众休息区等交流集聚地;D1展馆企业展示区,更有用餐区,特色小食区。为生物医药企业提供一站式采购服务。






 个人中心
个人中心
 我是园区
我是园区




