您还不是认证园区!
赶快前去认证园区吧!
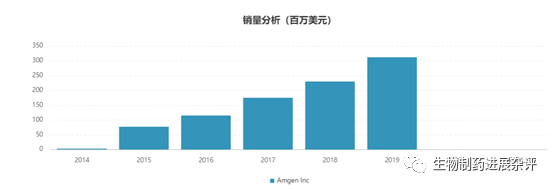
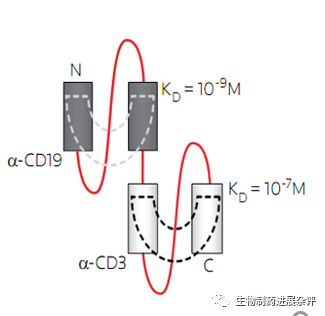
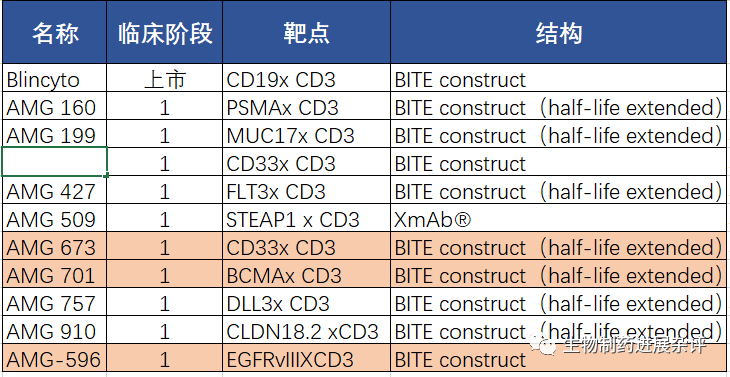
安进公司于2月2日下午公布了第四季度的数据,值得注意的是,安进宣布了暂时终止了3个双特异性抗体项目的临床研究,其中AMG 701(pavurutamab,BCMAXCD3靶点)的终止很意外。毕竟一度认为AMG 701为代表的双抗将会是CAR-T的终结者,结果没想到先把自己终结了。AMG 701终止的原因主要还是安全性因素,预计应该是CRS原因。
-
Dose escalation data for AMG 701 (pavurutamab), a half-life extended BiTE molecule targeting B-cell maturation antigen (BCMA) for relapsed or refractory multiple myeloma, were presented at the American Society of Hematology Annual Meeting in December. Enrollment in the Phase 1 study has been paused while we discuss protocol modifications to optimize safety monitoring and mitigation with the FDA. Currently enrolled patients who are demonstrating benefit may continue to receive investigational product and the Company expects to resume patient enrollment in H1 2021. -
Clinical development of AMG 673, a half-life extended BiTE molecule targeting CD33, is paused while we gather further information on the CD33 program through progression of AMG 330. -
Clinical development of AMG 596, a BiTE molecule targeting EGFR variant III for glioblastoma, has been stopped as we prioritize our portfolio.
药渡咨询郭雷团队




 个人中心
个人中心
 我是园区
我是园区




