▎药明康德内容团队编辑
4月份,根据PDUFA日程安排,FDA有可能在未来3周内对4款新药做出审评决定,其中有两款来自中国,详见下表。
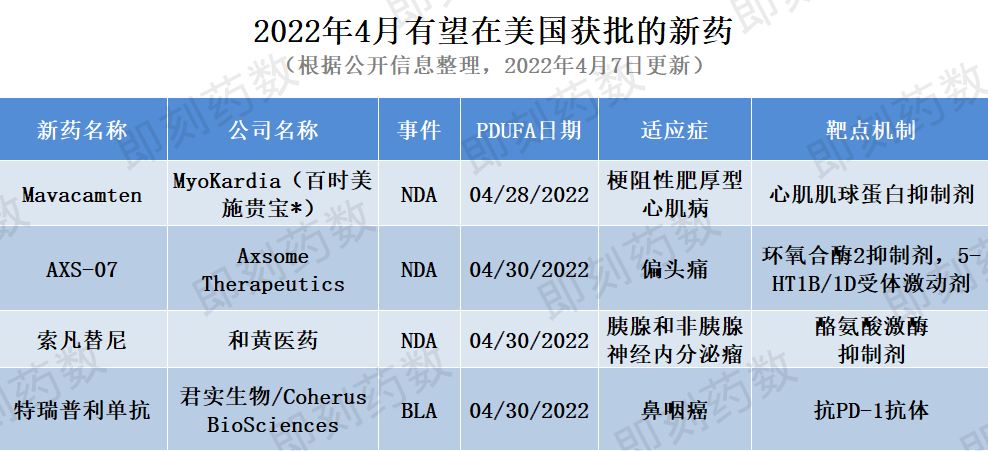
▲未来3周有望在美国获批的新药(药明康德内容团队制图,点击可见大图,*括号中公司合并了产品研发公司)
1.新药名称:mavacamten
公司名称:百时美施贵宝
适应症:梗阻性肥厚型心肌病

百时美施贵宝的mavacamten是该公司通过于2020年11月收购MyoKardia获得的口服心肌肌球蛋白抑制剂,用于口服治疗梗阻性肥厚型心肌病(oHCM)。Mavacamten于2021年3月提交的NDA被FDA基于积极的
3期
临床结果
接收,该结果表明主要和次要疗效终点均得到满足,安全性与安慰剂相似。如果在2022年4月28日的PDUFA日期前获得FDA批准,它可能成为针对oHCM疾病机理的潜在“first-in-class”药物。除了在美国的上市申请外,mavacamten在欧盟也有一个正在审查中的上市授权申请(Marketing Authorisation Application,MAA),用于相同的适应症。目前,该公司正在进行临床试验以评估mavacamten治疗其他适应症的潜在能力,包括非梗阻性肥厚型心肌病。
2.新药名称:AXS-07
公司名称:Axsome Therapeutics
适应症:偏头痛

Axsome专注于治疗中枢神经系统疾病,正在开发AXS-07,这是一种新型、快速吸收的口服固定剂量组合,它的活性成分为美洛昔康(meloxicam)和利扎曲普坦(rizatriptan),并使用Axsome公司的MoSEIC配方技术,用于偏头痛的急性治疗。两项3期试验的积极结果显示,与安慰剂和活性对照利扎曲普坦相比,该组合具有快速和持续的疗效。FDA于2021年9月接收了Axsome对AXS-07的NDA,将PDUFA目标日期设定为2022年4月30日。如果获得批准,该组合将提供一种治疗偏头痛的多机制方法。
3.新药名称:索凡替尼
公司名称:和黄医药
适应症:胰腺和非胰腺神经内分泌瘤

和黄医药的索凡替尼(surufatinib)是一种口服酪氨酸激酶抑制剂,具有抗血管生成和免疫调节双重活性,用于治疗胰腺和非胰腺神经内分泌瘤(NET)。基于积极的3期结果,Surufatinib的NDA申请于2021年6月被FDA接收,结果表明该药物已达到主要疗效终点。FDA已将PDUFA目标日期设定为2022年4月30日。该药物已在中国上市,也在寻求欧盟的批准。如果根据当前计划在美国获得批准,该公司预计该药将在2022年下半年上市,为晚期NET患者提供额外的治疗选择。
4.新药名称:特瑞普利单抗(toripalimab)
公司名称:君实生物/Coherus BioSciences
适应症:鼻咽癌

由君实生物和Coherus BioSciences联合开发的特瑞普利单抗(toripalimab)是一种抗PD-1单克隆抗体,用于治疗复发性或转移性鼻咽癌。2021年11月,FDA接收了toripalimab的生物制品许可申请(BLA),该药物得到2期和3期研究的积极安全性和有效性结果的支持,并被授予优先审评资格。PDUFA日期为2022年4月30日。特瑞普利单抗已获批在中国上市,用于治疗不可切除或转移性黑色素瘤。如果获得FDA的批准,特瑞普利单抗与化学疗法相结合,可能成为晚期鼻咽癌一线治疗的新标准。针对晚期鼻咽癌,目前尚无FDA批准的免疫肿瘤学疗法。
Expected US New Drug Approvals in April 2022
In April, the FDA’s schedule remains busy, with a potential of four drugs to be approved within the month.
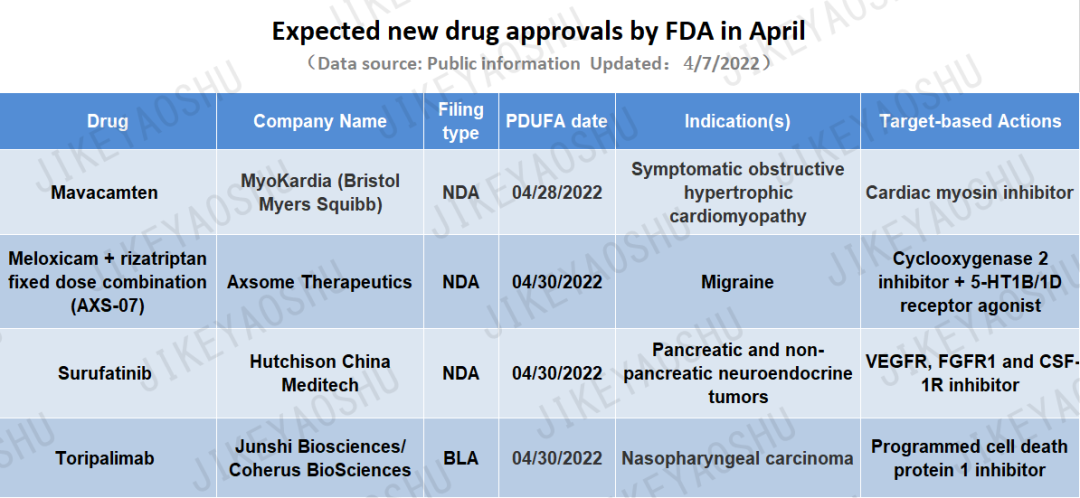
(By WuXi AppTec content team. Click the image to view at full size)
1. Drug: mavacamten
Company: Bristol Myers Squibb
Indication(s): Symptomatic obstructive hypertrophic cardiomyopathy

Bristol Myers Squibb’s mavacamten is an oral cardiac myosin inhibitor gained by the company via acquisition of MyoKardia in November 2020, for the potential oral treatment of symptomatic obstructive hypertrophic cardiomyopathy. Mavacamten’s NDA submission in March 2021 was accepted by the FDA based on positive phase III results which demonstrated that both the primary and secondary efficacy endpoints were met and the safety profile was similar to that of placebo. If approved by the FDA by the PDUFA date of April 28, 2022, it could become a first-in-class medicine targeting the underlying disease of symptomatic obstructive hypertrophic cardiomyopathy, a cardiovascular disease with serious unmet need. Other than the marketing application in the US, mavacamten also has a MAA under review in the EU for the same indication. Clinical trials are ongoing to evaluate the potential ability of mavacamten to treat additional indications including non-obstructive hypertrophic cardiomyopathy.
2. Drug: meloxicam + rizatriptan fixed dose combination (AXS-07)
Company: Axsome Therapeutics
Indication(s): Migraine

With a focus on therapies for the management of central nervous system disorders, Axsome is developing AXS-07, a novel, rapidly absorbed, oral fixed-dose combination of the non-steroidal anti-inflammatory COX2 inhibitor meloxicam and the 5-HT1B/D agonist rizatriptan, formulated using the company’s MoSEIC technology, for the acute treatment of migraine. The FDA accepted Axsome’s NDA for AXS-07 in September 2021, based on positive results from two phase III trials which showed rapid and sustained efficacy of the combination compared with placebo and the active comparator rizatriptan. The FDA has set the PDUFA target action date for April 30, 2022. If approved, the combination would provide a multi-mechanistic approach to treating migraine.
3. Drug: surufatinib
Company: Hutchison China Meditech (HUTCHMED)
Indication(s): Pancreatic and non-pancreatic neuroendocrine tumors

Another therapeutic which is seeking FDA approval is HUTCHMED’s surufatinib, a small molecule inhibitor of VEGFR and FGFR1 and CSF-1R tyrosine kinases, for the treatment of pancreatic and extra-pancreatic neuroendocrine tumors (NETs). Surufatinib’s NDA filing was accepted by the FDA in June 2021, based on positive phase III results which showed the drug to have met primary efficacy endpoint and was safe. The FDA has set a PDUFA target date as April 30, 2022. HUTCHMED has an ongoing expanded access program in the US with surufatinib for patients with limited treatment options for neuroendocrine tumor. According to the company’s recent announcement, clinical site inspections and pre-approval inspections of manufacturing facilities are in progress, with several already completed. Mid- and late-cycle review meetings with the FDA have also been completed. The drug is already available in China and is also seeking approval in the EU. If approved in the US under the current plan, the company anticipated launch by H2 2022 to provide additional treatment option for advanced NET patients.
4. Drug: toripalimab
Company: Junshi Biosciences/Coherus BioSciences
Indication(s): Nasopharyngeal carcinoma

Toripalimab is an anti-PD-1 monoclonal antibody being developed by Junshi and licensee Coherus, for the potential treatment of recurrent or metastatic nasopharyngeal carcinoma. In November 2021, the FDA accepted the toripalimab BLA, which is supported by positive safety and efficacy results from a phase II and a phase III studies and granted the BLA priority review, with a target action date of April 2022. Toripalimab was the first domestic anti-PD-1 monoclonal antibody approved for marketing in China to treat unresectable or metastatic melanoma. If approved by the FDA, toripalimab, in combination with chemotherapy, could become the new standard of care for first-line treatment of advanced nasopharyngeal carcinoma, an aggressive tumor that currently has no FDA-approved immuno-oncology treatment.
参考资料:
[1] Bristol Myers Squibb - U.S. Food and Drug Administration Approves First LAG-3-Blocking Antibody Combination, Opdualag™ (nivolumab and relatlimab-rmbw), as Treatment for Patients with Unresectable or Metastatic Melanoma. Retrieved March 28, 2022 from,
https
://news.bms.com/news/corporate-financial/2022/U.S.-
Food-and-Drug-Administration-Approves-First-LAG-3-Blocking-Antibody-Combination-Opdualag-nivolumab-and-relatlimab-rmbw-as-Treatment-for-Patients-with-Unresectable-or-Metastatic-Melanoma/default.aspx
[2] Marinus Pharmaceuticals, Inc. - Marinus Pharmaceuticals Announces FDA Approval of ZTALMY® (ganaxolone) for CDKL5 Deficiency Disorder. Retrieved March 28, 2022 from,
https://
ir.marinuspharma.com/news/news-details/2022/Marinus-Pharmaceuticals-Announces-FDA-Approval-of-ZTALMY-ganaxolone-for-CDKL5-Deficiency-Disorder/default.aspx
[3] BioXcel Therapeutics Reports Fourth Quarter and Full Year 2021 Financial Results and Recent Operational Highlights. Retrieved March 28, 2022 from,
https
://
ir.bioxceltherapeutics.com/news-releases/news-release-details/bioxcel-therapeutics-reports-fourth-quarter-and-full-year-2021
[4] BioXcel Therapeutics Announces Journal of the American Medical Association Publication of Data from SERENITY II Pivotal Phase 3 Trial Evaluating BXCL501 in Bipolar Disorders. Retrieved March 28, 2022 from,
https
://
ir.bioxceltherapeutics.com/news-releases/news-release-details/bioxcel-therapeutics-announces-journal-american-medical
[5] Vutrisiran factsheet. Retrieved March 28, 2022 from,
https
://
www.alnylam.com/wp-content/uploads/pdfs/Vutrisiran-Fact-Sheet.pdf
[6] Alnylam Announces U.S. Food and Drug Administration Acceptance of New Drug Application for Investigational Vutrisiran for the Treatment of the Polyneuropathy of Hereditary ATTR Amyloidosis. Retrieved March 28, 2022 from,
https://
investors.alnylam.com/press-release?id=25811
[7] Fourth Quarter and Full Year 2021 Financial Results presentation - Alnylam. Retrieved March 28, 2022 from,
https
://
alnylampharmaceuticalsinc.gcs-web.com/static-files/a66094b4-18cb-4d0e-9832-18e62e242f97
[8] Bristol Myers Squibb Completes Acquisition of MyoKardia, Strengthening Company’s Leading Cardiovascular Franchise. Retrieved March 28, 2022 from,
https
://
news.bms.com/news/details/2020/Bristol-Myers-Squibb-Completes-Acquisition-of-MyoKardia-Strengthening-Companys-Leading-Cardiovascular-Franchise/default.aspx
[9] U.S. Food and Drug Administration (FDA) Accepts Bristol Myers Squibb’s Application for Mavacamten in Symptomatic Obstructive Hypertrophic Cardiomyopathy (oHCM). Retrieved March 28, 2022 from,
https
://news.bms.com/news/corporate-financial/2021/U.S.-
Food-and-Drug-Administration-FDA-Accepts-Bristol-Myers-Squibbs-Application-for-Mavacamten-in-Symptomatic-Obstructive-Hypertrophic-Cardiomyopathy-oHCM/default.aspx
[10] Efficacy and Safety of Mavacamten in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy: Results of the EXPLORER-HCM Study. Retrieved March 28, 2022 from,
https
://www.ajmc.com/view/adults-with-symptomatic-obstructive-hypertrophic-cardiomyopathy-results-of-the-explorer-hcm-study
[11] Bristol Myers Squibb Q4 2021 Results presentation. Retrieved March 28, 2022 from,
https
://
s21.q4cdn.com/104148044/files/doc_presentations/2021/BMY-2021-Q4-Results-Investor-Presentation.pdf
[12] A Long-Term Safety Extension Study of Mavacamten in Adults Who Have Completed MAVERICK-HCM or EXPLORER-HCM. Retrieved March 28, 2022 from,
https
://www.clinicaltrials.gov/ct2/show/NCT03723655?term=Mavacamten&draw=2&rank=7
[13] A Study of Mavacamten in Participants With HFpEF and Elevation of NT-proBNP With or Without Elevation of cTnT (EMBARK-HFpEF). Retrieved March 28, 2022 from,
https
://
www.clinicaltrials.gov/ct2/show/NCT04766892?term=Mavacamten&draw=2&rank=3
[14] European Medicines Agency Validates Bristol Myers Squibb’s Application for Mavacamten for the Treatment of Obstructive Hypertrophic Cardiomyopathy. Retrieved March 28, 2022 from,
https
://
news.bms.com/news/details/2021/European-Medicines-Agency-Validates-Bristol-Myers-Squibbs-Application-for-Mavacamten-for-the-Treatment-of-Obstructive-Hypertrophic-Cardiomyopathy/default.aspx
[15] Corporate Presentation January 2022 - Axsome Therapeutics. Retrieved March 28, 2022 from,
https
://
axsometherapeuticsinc.gcs-web.com/static-files/d0beb034-c48d-45c2-8ac3-bfd85f26177f
[16] Axsome Therapeutics Announces FDA Acceptance of New Drug Application for AXS-07 for the Acute Treatment of Migraine. Retrieved March 28, 2022 from,
https://axsometherapeuticsinc.gcs-web.com/news-releases/news-release-details/axsome-therapeutics-announces-fda-acceptance-new-drug-0
[17] Axsome Therapeutics Reports Fourth Quarter and Full Year 2021 Financial Results and Provides Business Update. Retrieved March 28, 2022 from,
https://
axsometherapeuticsinc.gcs-web.com/news-releases/news-release-details/axsome-therapeutics-reports-fourth-quarter-and-full-year-2021
[18] Evaluation of surufatinib, an orally available VEGFR, FGFR1 and CSF-1R inhibitor, in combination with immune checkpoint blockade or chemotherapy in preclinical tumor models. Retrieved March 28, 2022 from,
https://www.ejcancer.com/article/S0959-8049(20)31132-1/fulltext
[19] HUTCHMED Reports 2021 Full Year Results and Provides Business Updates. Retrieved March 28, 2022 from,
https
://www.hutch-med.com/fy2021-results/#_
edn3
[20] U.S. FDA Accepts Filing of HUTCHMED’s NDA for Surufatinib for the Treatment of Advanced Neuroendocrine Tumors. Retrieved March 28, 2022 from,
https
://www.hutch-med.com/fda-accepts-surufatinib-nda-for-net
/
[21] Expanded Access Program of Surufatinib. Retrieved March 28, 2022 from,
https://www.clinicaltrials.gov/ct2/show/NCT04814732
[22] US FDA Accepted BLA Filing for Toripalimab for Treatment of NPC. Retrieved March 28, 2022 from,
https://www.junshipharma.com/en/News.html
[23] Efficacy, Safety, and Correlative Biomarkers of Toripalimab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma: A Phase II Clinical Trial (POLARIS-02). Retrieved March 28, 2022 from,
https://ascopubs.org/doi/full/10.1200/JCO.20.02712
[24] Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Retrieved March 28, 2022 from,
https://www.nature.com/articles/s41591-021-01444-0













 个人中心
个人中心
 我是园区
我是园区




