有观点,有态度
这是医业观察的第1610-6期文章
来源:康立明生物
2022年11月22日,康立明生物与领先制药集团Cooper Pharma的子公司Cooper Biosciences MENA建立合作,开拓康立明生物的拳头产品(长安心®)在摩洛哥、埃及、沙特阿拉伯和阿联酋市场业务。长安心®是国内率先获批上市的肠癌粪便基因检测产品,具有精准、便捷、无创、早诊的优点。“长安心”目前已进入全国约740家医疗机构,在临床、大健康、公共卫生领域被广泛使用。截止今年10月份数据,长安心®在国内共完成约52万例实际检测,获得医生与受检者的广泛好评。
Cooper Pharma是一家创始于1933年的生物制药集团,总部设在摩洛哥卡萨布兰卡,业务覆盖非洲和中东等地区。集团通过附属子公司Cooper Biosciences MENA在中东和北非地区推广和分销创新型疗法和体外诊断产品。
“我们非常自豪地将长安心®引入我们的地区,这将解决一个重要且未满足的临床需求。我们相信,这种创新的IVD产品将在中东和北非的癌症早期检测中发挥重要作用。”Cooper Biosciences MENA首席执行官Ayman Cheikh Lahlou表示。
此次合作将通过Cooper Pharma的现有市场覆盖架构,首先在摩洛哥和沙特阿拉伯推动长安心®市场准入,并实现产品本地化检测服务和支持。
目前,长安心®已在亚太市场进行销售推广,这次与Cooper Pharma的合作标志着长安心®在中东和北非市场开拓的第一步。康立明生物高级副总裁廖吉弘先生对此次合作进行展望:“我非常高兴能与Cooper Pharma建立合作关系,将会取得双赢的成果。基于Cooper Pharma在药品和IVD产品分销及市场拓展的卓越能力,我坚信,能够为医疗专业人士提供的一流IVD产品,其临床性能将在改善中东和北非的癌症筛查实践中发挥重要作用。我们希望通过双方的长期合作,实现挽救更多的生命的愿景。”
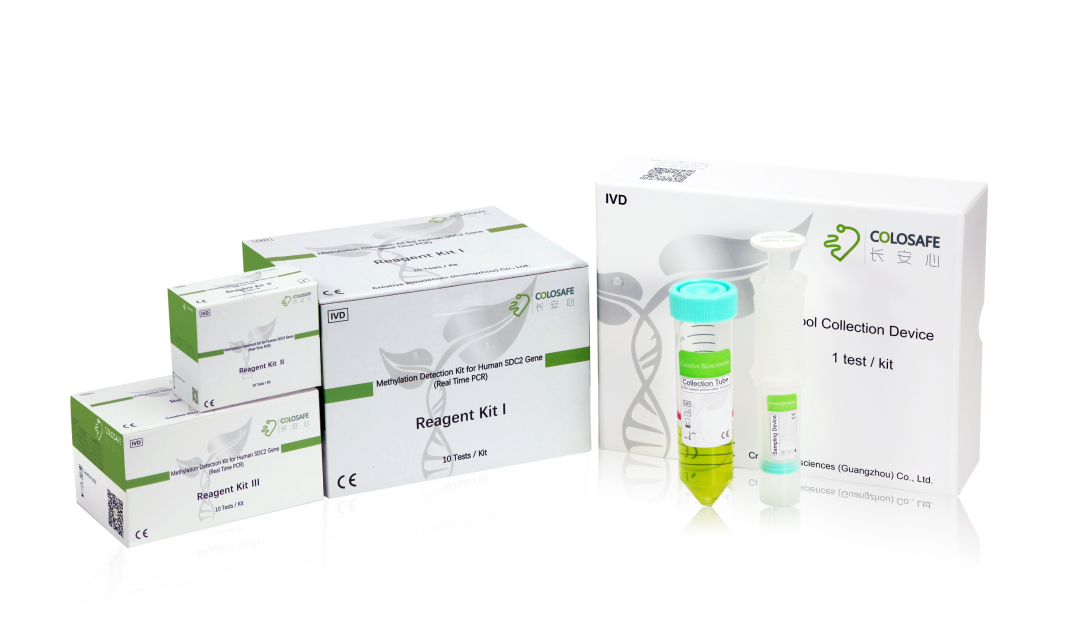
关于长安心®
长安心®(人类肠癌SDC2粪便基因检测试剂盒)于2018年11月20日获国家药监局颁发的三类医疗器械注册证书,是我国率先获批上市的肠癌粪便基因检测试剂盒,成为肠癌无创基因检测的破冰之作。长安心®通过检测粪便中的脱落细胞的肿瘤标志物,来精准判断受检者是否可能患有结直肠癌或者癌前腺瘤,帮助医生发现患者的癌前病变和早期结直肠癌,将结直肠癌阻断在发生前或者早期阶段,从而达到预防和根治结直肠癌的目的。长安心®具有无创、精准、便捷等优点,目前已获得ISO13485认证和CE标志,符合欧盟安全、健康和环境要求。

关于康立明生物
广州康立明生物科技股份有限公司是由优秀科学家团队创办的一家高科技生物公司。公司于2015年1月登记注册,专注于长安心®粪便DNA肠癌检测试剂盒等肿瘤早期筛查诊断产品以及相关自动化检测设备的研发、生产和销售,并可提供相应的检测服务。公司拥有国际前沿的科研能力和众多独立的知识产权,未来还将继续加强,并将成果转化为成生产力。除了长安心®肠癌检测产品之外,公司还在开发肺癌、膀胱癌、宫颈癌、肝癌等肿瘤的早期筛查诊断产品和即时检测设备系统,并已取得令人惊喜的阶段性成果。公司本着“人类健康,我之使命”的崇高理想,力争在慢性病尤其肿瘤的早诊早治、预后监测等领域取得突破和发展,最终造福人民。

关于Cooper Pharma
Cooper Pharma是一家始创于1933年的生物制药集团,总部设在摩洛哥卡萨布兰卡,业务覆盖非洲和中东等地区。集团通过附属子公司与9个生产基地,以最优价格生产和销售最高质量的授权创新药物。集团产品线涵盖300多种产品系列,主要治疗领域包括: 消化科、肿瘤科、心脏科、皮肤科、风湿病科等。
Cooper Biosciences MENA为Cooper Pharma集团旗下附属子公司,致力于在中东和北非地区推广和分销创新型疗法和体外诊断产品。
Creative Biosciences & Cooper Biosciences MENA Announce Partnership for the Commercialization of Colosafe® in the MENA region
Guangzhou, China. November 22, 2022 -- Creative Biosciences (CreativeBio) and Cooper Biosciences MENA (Cooper) - an affiliate of Cooper Pharma Group announced today a partnership for the commercialization of Colosafe® in Morocco, Egypt, Saudi Arabia (KSA) and the United Arab Emirates (UAE). Colosafe® is CreativeBio’s flagship product which is the first NMPA approved stool DNA test kit for colorectal cancer. With the advantages of noninvasive, convenient, accurate and early detection of CRC, Colosafe® has been widely used in hospital, non-hospital, and government organized screening covering 740 medical institutions across the country. By October 2022, Colosafe® has performed over 520,000 testings in China, which has gained tremendous clinical recognition by the professionals and experts.
Cooper Pharma is a leading Moroccan pharmaceutical company based in Casablanca since 1933 and operating in Africa and the Middle East. The group is promoting and distributing innovative drugs and diagnostic solutions through its affiliate Cooper Biosciences MENA.
”We are very proud to bring Colosafe® to our region which will address an important unmet need. We are confident that this innovative IVD product will play an important role in cancer early detection in the Middle East and North Africa”,commented Ayman Cheikh Lahlou, Chief Executive Officer of Cooper.
The partnership will initially launch Colosafe® in Morocco and Saudi Arabia (KSA) using Cooper’s various established commercial channels. Cooper will market Colosafe® and perform testing in laboratories to offer localized service and support.
Colosafe® is currently marketed across the Asia Pacific, and the partnership with Cooper marks Colosafe’s initial launch in the Middle East and North Africa (MENA region). Mr. Leo Liao, Senior Vice President of CreativeBio, expect for this cooperation: "I am very pleased to establish this partnership with Cooper, which will be a win-win cooperation for both parties. Based on Cooper's outstanding expertise in the distribution and marketing of pharmaceutical and IVD products, I believe that our best-in-class IVD products will play an important role in improving the clinical practice of cancer screening in the Middle East and North Africa. We hope to save more lives through our long-term partnership."

About Colosafe®
Full name of Colosafe® is Methylation Detection Kit for Human SDC2 Gene (Real time PCR) & Stool Collection Device which is the first NMPA approved stool DNA test kit for colorectal cancer. Colosafe® entered the special approval channel of National Medical Products Administration in March 2017 and was cleared by NMPA on Nov 20, 2018. It is a product that can precisely detect and interpret the mutated messages (human SDC2 methylation) from stool for detection of colorectal cancer to help physicians to figure out the cancerous lesion early and prevent the development of CRC at early stage. Colosafe® has the advantages of noninvasive, convenient, accurate and early detection of CRC. It has obtained ISO13485 certification and CE mark complying with EU safety, health, and environmental requirements.

About CreativeBio
Creative Biosciences is an advanced biotech enterprise founded by a team of excellent scientists. Since its registration in January 2015, CreativeBio has been dedicated to the research and development of Colosafe®, an sDNA test for the early detection of colorectal cancer, as well as in manufacturing, sales, and its related automation equipment. The company not only possesses independent intellectual property rights but also boasts its world's top-notch R&D capability which will be improved and transformed into productivity in the future. On top of Colosafe®, other noninvasive early detection kits for lung, bladder, cervical, and liver cancers, together with POCT products have reached amazing development. Guided by the ultimate goal of “Human Health, My Mission”, CreativeBio is striving to make breakthroughs in early diagnosis, early intervention, and prognosis monitoring of chronicle diseases specifically cancer, to benefit all nations.

About Cooper
Based in Casablanca since 1933, Cooper Pharma is a leading Moroccan pharmaceutical company operating in Africa and the Middle East. Through its affiliates and 9 Manufacturing sites, Cooper Pharma is active in the production and marketing of branded generics and innovative drugs of the highest quality at the best price. The product portfolio of Cooper Pharma has nearly 300 presentations covering the main therapeutic areas: Gastroenterology, Oncology, Cardiology, Dermatology, Rheumatology, Clinics & Hospitals.
Cooper Biosciences MENA is an affiliate company dedicated to the promotion and distribution of innovative Therapeutics and Diagnostics products in the MENA region.

关于长安心®
长安心®(人类肠癌SDC2粪便基因检测试剂盒)于2018年11月20日获国家药监局颁发的三类医疗器械注册证书,是我国率先获批上市的肠癌粪便基因检测试剂盒,成为肠癌无创基因检测的破冰之作。目前已进入700多家医疗机构,其中含300多家三甲医院应用,赢得各临床科室、检验科及健康管理科等相关使用科室专家和受检者一致好评。
粪便DNA检测对于检测肠道肿瘤具有天然的优势,因为正常成人每天都会有上皮细胞脱落至肠腔并随粪便排出体外,而结直肠癌肿瘤细胞由于异常增殖,细胞与细胞间或者细胞基底膜的黏附性降低等因素,比正常上皮细胞更易脱落。因此,肠道肿瘤患者的粪便中会含有大量的从肠道肿瘤表面脱落的携带了肠癌病变信息的细胞和细胞成分,这些信息可以由特殊的检测手段来解读。长安心®正是一款可以准确解读粪便中基因异常改变(人类SDC2基因甲基化)的大肠癌检测工具,它可以帮助医生早期发现患者的肠癌病变情况,有希望将肠癌阻断在早期阶段,从而达到预防和根治肠癌的目的。
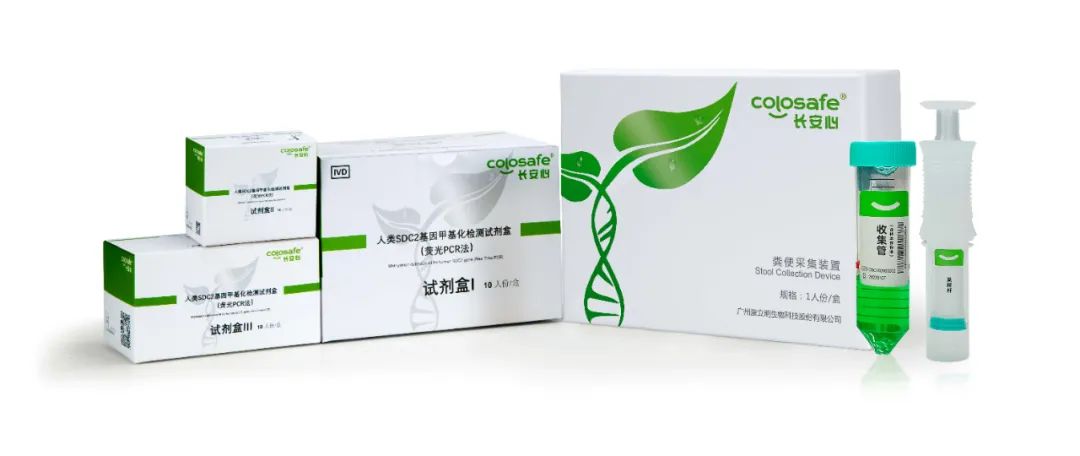
广州康立明生物科技股份有限公司是由优秀科学家团队创办的一家高科技生物公司。公司于2015年1月成立,专注于长安心®肠癌粪便基因检测试剂盒等肿瘤早期检测产品以及相关自动化检测设备的研发、生产和销售,并提供相应的检测服务。康立明生物总部落户于广州高新技术产业开发区,包括研发实验室、GMP生产车间、综合办公区等;在广州、天津、武汉、济南等地搭建了第三方医学检验实验室,总面积超过18000平方米。此外,于2020年3月份收购好芝生物公司,综合研发和团队力量进一步加强。公司拥有国际前沿的科研能力和众多独立的知识产权,未来还将继续加强,并将成果转化为生产力。除了长安心®肠癌粪便基因检测产品之外,公司还在开发肺癌、膀胱癌、宫颈癌、肝癌等肿瘤的无创检测产品和即时检测设备,并已取得令人惊喜的阶段性成果。目前,康立明生物已完成多轮优质风险投资,并获得了各级政府的大力支持,已成为行业内备受关注的明星企业。我们本着“人类健康,我之使命”的崇高理想,力争在慢性病尤其肿瘤的早诊早治、预后监测等领域取得突破和发展,持续造福人民。
星哥聊医疗视频号
直播预告
The End


欢迎点击关注
喜欢就一键三连,点赞,在看,分享!



 个人中心
个人中心
 我是园区
我是园区





