
3月10日,康希诺生物股份公司(以下简称“康希诺生物”,康希诺-U 688185.SH,康希诺生物-B 06185.HK)在医学生物类论文预印本平台medRvix上发表了题为《Aerosolized Ad5-nCoV booster vaccination elicited potent immune response against the SARS-CoV-2 Omicron variant after inactivated COVID-19 vaccine priming》的学术论文。
结果显示,序贯加强康希诺生物腺病毒载体新冠疫苗克威莎(吸入或肌注剂型)后,抗体水平优于灭活疫苗同源加强及重组蛋白疫苗序贯加强,可显著激发细胞免疫反应,降低奥密克戎突变株传播和感染,有效防止重症发生及突变病毒的逃逸。康希诺生物腺病毒载体疫苗克威莎或为序贯加强更优选择。
本次试验将904名已接种2剂灭活疫苗满6个月的受试者随机分为四组,分别以1剂肌注克威莎、克威莎吸入剂型(吸入剂量为肌注剂量的1/5)、重组蛋白疫苗进行序贯加强,以及1剂灭活疫苗进行同源加强。
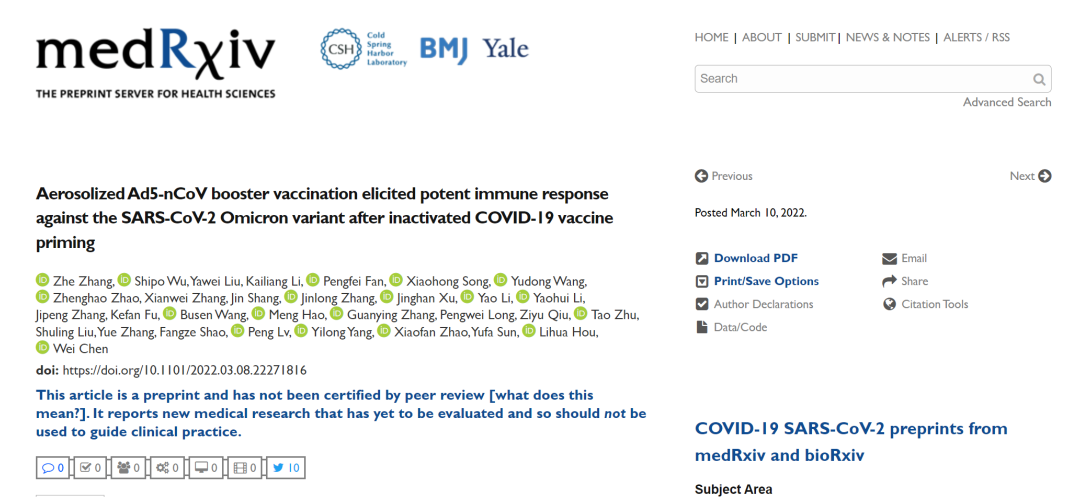
免疫原性结果显示,序贯加强克威莎所诱导的RBD特异性抗体水平,明显高于重组蛋白疫苗序贯加强或灭活疫苗同源加强。加强14天后,吸入剂型组的RBD抗体水平为523 (IQR, 137-1336),肌注克威莎为464 (IQR, 210-1097),重组蛋白疫苗及灭活疫苗分别为174 (IQR, 58-488)、 61 (IQR, 30-124)。
A:受试者新冠病毒RBD特异性抗体的几何平均浓度。B:受试者加强免疫前后对RBD特异性结合抗体反应因子的变化。
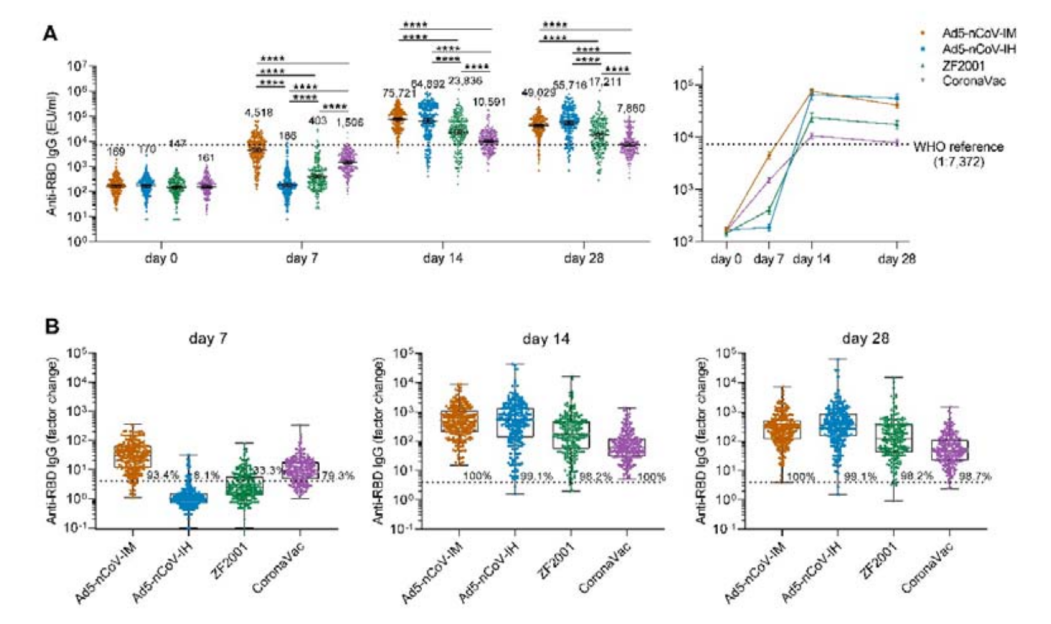
数据显示,采用吸入剂型加强28天后,对原始毒株的中和抗体GMTs为874 (95% CI = 569-1342),肌注克威莎为628 (95% CI = 455-868) ,重组蛋白疫苗和灭活疫苗组分别下降到210(95%CI=137-321)和69(95%CI=51-93)。说明序贯加强腺病毒载体新冠疫苗克威莎具有显著优势。
序贯加强克威莎除可显著增强对原始毒株的中和抗体水平外,还可有效中和奥密克戎突变株。加强14天后,克威莎吸入剂型组的抗体水平320 (95% CI = 191-538),肌注克威莎组为261 (95% CI = 178-382),重组蛋白疫苗组和灭活疫苗组分别为86 (95% CI = 59-127)、 54 (95% CI = 42-71)。
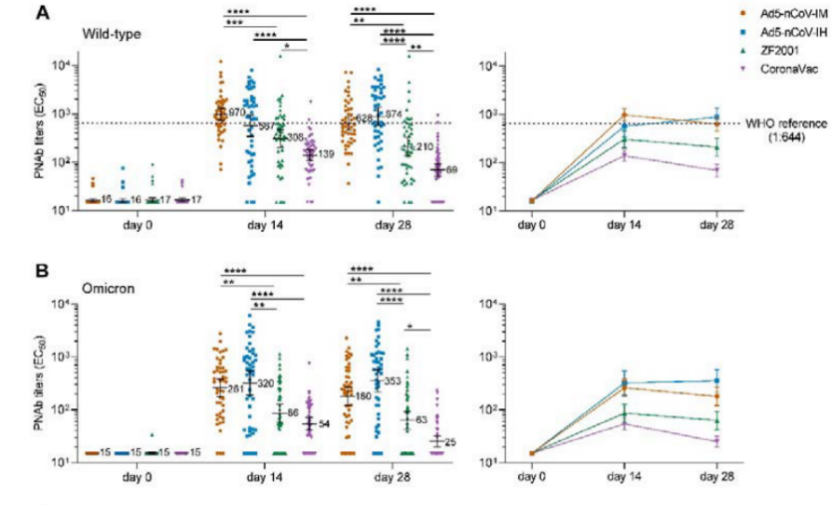
受试者对新冠病毒野生型 (A)或Omicron突变株(B)的几何平均滴度。
新冠病毒刺突蛋白特异性IgG B细胞反应结果显示,序贯加强的中和抗体水平比同源加强高,其中吸入剂型效果最优,可显著促进免疫反应,提高受试者的免疫应答。
除了可显著提升抗体水平外,序贯加强克威莎还可显著诱导细胞免疫反应。在第14天和第28天,吸入剂型IFN-γ的阳性率为100%(95%CI,92.6%~100.0%)和95.7%(95%CI,85.2%~99.5%),肌注剂型为85.4% (95% CI, 72.2%-93.9%) 和 68.8% (95% CI, 53.7%-81.3%)。结果显示吸入剂型细胞免疫反应最强,显著优于重组蛋白疫苗组(<25%)和灭活疫苗组(<42%)。
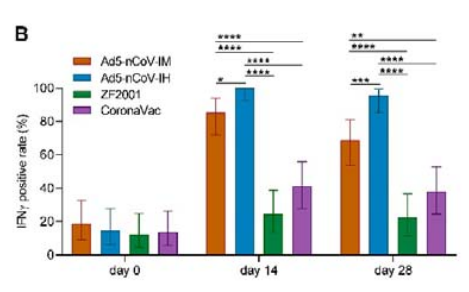
受试者新冠病毒刺突蛋白特异性IFN-γ阳性率。
当前,我国疫情呈现多点散发和局部聚集性暴发态势,多地疫情由奥密克戎突变株引发,其传染性强、传播速度快等特点令疫情防控难度进一步加大。上述研究结果证明,采用康希诺生物腺病毒载体新冠疫苗克威莎进行序贯加强可提升中和抗体水平,对奥密克戎突变株也具有较好的中和能力,且显著高于重组蛋白疫苗和灭活疫苗,有效防止病毒逃逸。
序贯加强克威莎可同时激发体液免疫和细胞免疫,无论是肌注还是吸入剂型,免疫效果显著优于以重组蛋白疫苗和灭活疫苗进行加强。同时,采用吸入剂型克威莎进行序贯加强,仅需肌肉注射剂量的1/5,除可诱导显著的全身免疫反应外还可诱导高效的黏膜免疫,以达到三重保护的效果。
可点击下方“阅读原文”,查看文章。

关于康希诺生物
康希诺生物股份公司(H股简称:康希诺生物-B,代码06185.HK;A股简称:康希诺-U,代码688185.SH),2009年成立于中国天津,致力于为中国及全球公共卫生研发、生产和商业化创新疫苗。公司现有五个创新疫苗平台技术,包括基于病毒载体技术、合成疫苗技术、蛋白结构设计和重组技术、mRNA技术和制剂及给药技术。目前,公司已建立覆盖12种传染病的17种疫苗的强大研发管线,其中包括2021年获批附条件上市的重组新型冠状病毒疫苗(5型腺病毒载体),A群C群脑膜炎球菌多糖结合疫苗(CRM197载体)和ACYW135群脑膜炎球菌多糖结合疫苗(CRM197载体)。
Latest Study Shows Advantages of CanSinoBIO’s Convidecia as Heterologous Booster against Omicron Variant
Tianjin, March 14, 2022 – CanSino Biologics Inc. (“CanSinoBIO”) (SSE: 688185, HKEX: 06185) today announced that medRxiv, a preprint platform for preliminary biomedical research, published an academic paper on the efficacy of CanSinoBIO’s Recombinant COVID-19 Vaccine (“Ad5-nCoV”, trade name: Convidecia) as a heterologous booster against the Omicron variant. The results showed that booster vaccination with either intramuscular injection or inhaled version of Convidecia generated greater neutralizing antibody responses than those induced by the homologous inactivated vaccine booster or heterologous recombinant protein vaccine booster. A heterologous booster with one dose of Convidecia can significantly increase cellular immune protection against the transmission of and infection with the Omicron variant, and effectively prevent severe cases and immune escape.
Study Overview and Results
The study was open and parallel-controlled, with 904 participants primed with two doses of inactivated vaccines six months prior and were randomly assigned to four groups to receive one of the following boosters: one dose of Convidecia via intramuscular injection, one dose of the inhaled version of Convidecia (one fifth dosage compared to the intramuscular injection), one dose of recombinant protein subunit vaccine, and one dose of inactivated vaccine.
The study showed that boosting with either intramuscular or inhaled version of Convidecia can elicit significantly higher RBD-specific binding antibodies than those induced by recombinant protein vaccine or inactivated vaccine. 14 days following the booster vaccination, the RBD-specific antibody levels of participants were 523 (IQR, 137-1336) for the group of Convidecia by inhalation, and 464 (IQR, 210-1097) for the group of Convidecia by intramuscular injection. The results were significantly higher than the RBD-specific antibody levels of 174 (IQR, 58-488) for the group that received the recombinant protein vaccine, and 61 (IQR, 30-124) for the group of inactivated vaccine.
Strong Neutralizing Antibody Responses Against the Omicron Variant
The study observed an increased neutralizing antibody response against the wild-type SARS-CoV-2 induced by the inhaled version of Convidecia. At day 28 following the booster vaccination, the study showed an increased geometric mean titer (“GMT”) of 874 (95% CI = 569-1342) for the group of Convidecia by inhalation, 628 (95% CI = 455-868) for the group of Convidecia by intramuscular injection, while the GMTs of those in the recombinant protein vaccine and inactivated vaccine groups decreased to 210 (95% CI = 137-321) and 69 (95% CI = 51-93), respectively.
Importantly, Convidecia also demonstrated proven protection against the Omicron variant. The two groups who received Convidecia as a booster exhibited significantly higher immune responses, with a GMT of 320 (95% CI = 191-538) for the inhaled group and a GMT of 261 (95% CI = 178-382) for the intramuscular group 14 days after booster vaccination. By comparison, the GMT results of the recombinant protein vaccine and inactivated vaccine groups were 86 (95% CI = 59-127) and 54 (95% CI = 42-71), respectively.
In addition, administering Convidecia as a heterologous booster can significantly induce cellular immune responses. Notably, the inhaled version of Convidecia induced the strongest IFN-γ response among the four study groups, at 100% (95% CI, 92.6%-100.0%) and 95.7% (95% CI, 85.2%-99.5%) at day 14 and 28 following booster vaccination, respectively. The results were higher than the intramuscular Convidecia group, which were 85.4% (95% CI, 72.2%-93.9%) and 68.8% (95% CI, 53.7%-81.3%) , respectively.
Overall, the latest study showed that administering CanSinoBIO’s Convidecia as a heterologous booster can induce stronger immune response than the recombinant protein and inactivated vaccines, stimulating greater humoral and cellular immune responses regardless of delivery route. Additionally, the inhaled version of Convidecia provides a more effective and efficient alternative for the booster vaccination program, as it can also induce mucosal immunity to achieve triple protection with only one fifth dosage of an injectable version of Convidecia.





 个人中心
个人中心
 我是园区
我是园区




