
△ 写意巨献,1个主论坛,5个分会场,9个主题论坛,
数十位CMO齐聚
,千人大会等你来看!
作者:李杰教授
翻译:同写意团队

杰克说药是著名药史专家Jie Jack Li(李杰)教授专为同写意打造的药林外史精品专栏,将讲述一个个药物发现背后的故事。李杰教授现为上海睿智的副总裁,先后出版了30本有机和药物化学方面的书籍以及药物发现史,其中10本与诺奖得主E. J. Corey合作完成。其《Blockbuster Drugs》一书获 2015 Alpha Sigma Nu Science Book 奖,并被翻译成中文出版,深受欢迎。
开设写意专栏,请联系同写意秘书处(微信号tongxieyimishuchu)

最近,FDA完成了罗沙司他(roxadustat)的审查。如果获批,由FibroGen和阿斯利康共同开发的罗沙司他将成为首个在美国上市的脯氨酰羟化酶抑制剂用于治疗由慢性肾病引起的贫血症。不幸的是,在2021年8月11日,根据其咨询委员会的建议,FDA出于安全考虑拒绝了罗沙司他。
与此同时,FDA要求FibroGen和阿斯利康重新再做一项安全性的临床试验, 然后才会重新考虑再来审查罗沙司他。对于罗沙司他这个药, 这可能是最坏的结果。因为如果没有罗沙司他在美国的收入,这两个公司的投资者不会愿意投资又一项昂贵的Ⅲ期临床试验。
FibroGen本已四面楚歌的股价在这消息传出后继续下跌,目前股价不到13美元,尽管今年2月份该股曾超过52美元。
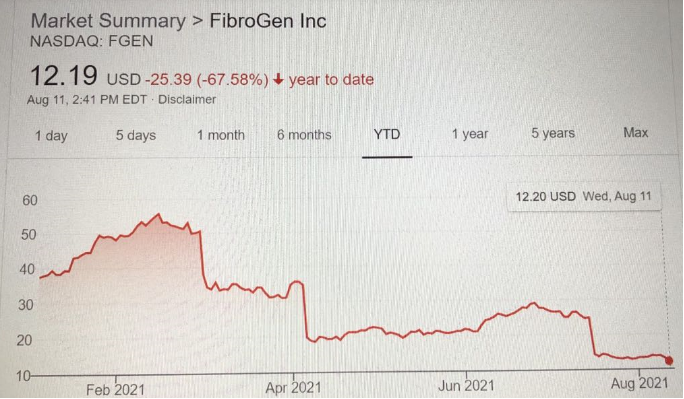
有趣的是,自2018年以来,罗沙司他(爱瑞卓)在中国和日本市场上一直用于治疗肾性贫血症。
为什么呢?
人体内有三种血细胞:红细胞、白细胞和血小板。血浆占血液总量的55%。红细胞约占血液体积的45%, 它们将氧气从肺部输送到身体的其他部位。白细胞保护我们免受细菌和病毒的侵袭;血小板(血液中不到1%)是黏性小细胞碎片,帮助血液凝固。
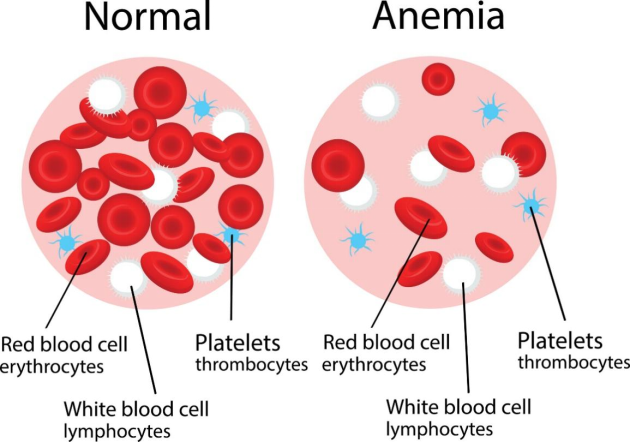
贫血症是血液循环中红细胞或血红蛋白减少的一种疾病。血红蛋白是红细胞中的一种蛋白质,它含有一个与血红素结合的铁原子,负责向全身细胞运送氧气。

慢性肾病、癌症、慢性炎症、营养和遗传缺陷都会引起贫血。贫血症在患有慢性肾病的患者中尤为普遍。慢性肾病可能由糖尿病、高血压、肥胖、吸烟等多种因素引起。这个病通常是个慢性病, 在全世界有8.4亿患者。其特征是肾功能逐渐丧失,最终可能导致肾衰竭。该疾病经常导致明显的疲劳、认知功能障碍和生活质量下降,并导致住院、心血管并发症和死亡的风险的增加。
治疗由慢性肾病引起的贫血症的老的方法包括输血、补铁和维生素。但这些方法的疗效有限。今天标准的治疗方法是用促红细胞生成素。安进和强生生产的Epogen和Procrit是两种市上常见的的促红细胞生成素。
脯氨酰羟化酶(PHD)抑制剂,例如罗沙司他,在正常条件下可以稳定缺氧诱导因子(HIF)并诱导红细胞生成素的产生[1]。这类药代表了治疗由慢性肾病引起的贫血症的一种新的方法。
低氧能够诱导缺氧诱导因子刺激促红细胞生成素产生来促进红细胞的合成。缺氧诱导因子于1993年首次发现。[2]1999年,von Hippel−Lindau肿瘤抑制蛋白(pVHL)被证实在HIF-1a氧依赖蛋白水解中发挥关键作用。[3]HIF-1α被E3泛素连接酶识别,泛素连接酶通过泛素化标记HIF-1α并将其带到蛋白酶体进行降解。这个过程的原理与使用泛素-蛋白酶体系统(UPS)的PROTACs相同。
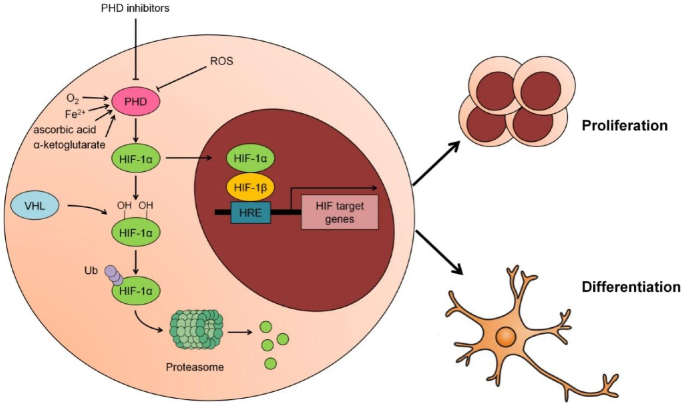
另一方面,脯氨酰羟化酶结构域抑制可以稳定缺氧诱导因子。缺氧诱导因子的稳定也会降低肝源激素铁调素——调节铁原子的稳态的激素。许多口服的脯氨酰羟化酶抑制剂,如罗沙司他、达产司他(daprodustat)、地沙司他(desidustat)和瓦达司他(vadadustat)都处于晚期临床试验阶段。
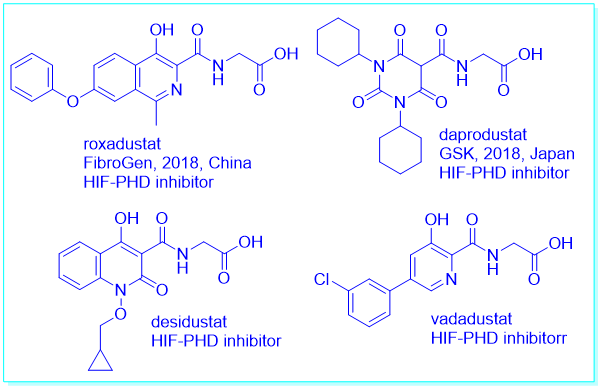
作为一种先驱的脯氨酰羟化酶抑制剂,罗沙司他对肾性贫血具有包括促进红细胞生成素的产生和改善铁代谢的协同作用。
FibroGen的罗沙司他可逆地结合和并有效地抑制脯氨酸羟化酶,减少HIF-1α的分解和促进HIF的转录活性。[4]它是一种口服活性药物,因此优于注射用的促红细胞生成药物。Epogen和Procrit也会增加凝血事件的风险,因此并没有被批准用于非透析依赖患者。
罗沙司他在中国、日本、智利和韩国已被批准用于透析依赖和非透析依赖患者的慢性肾脏病贫血。在日本和其他一些市场,它由安斯泰来销售。[5]罗沙司他在欧洲也被推荐批准上市。
在FDA的审查过程中,对罗沙司他的有效性没有任何疑问,但担心其安全性:主要与严重血栓栓塞事件的风险有关。在深入研究了临床试验数据后,FDA审查员认为,在透析依赖人群中,与促红细胞生成素治疗相比,在非透析依赖人群中与安慰剂相比,罗沙司他与死亡、血栓、严重感染等风险增加有关。
在FDA内部审查文件披露了大量投资者此前不知道的新的安全性问题后,在今年7月的会议上,咨询委员会以13-1的投票结果反对罗沙司他在非透析患者中的批准,12-2的投票结果反对透析依赖患者的批准。对安全性数据错误“直接负责”的FibroGen科学家立即被解雇。
现在, 每个人心中的问题是:罗沙司他的安全性问题是基于这个分子本身?或基于作用机制?我个人当然希望是前者。
如果是前者,那么罗沙司他的延迟为其它口服羟化酶抑制剂类的竞争对手, 例如葛兰素史克的达普罗司他(daprodustat), 在美国市场提供赶上罗沙司他的机会。达普罗司他的Ⅲ期临床试验的资料解析将于今年晚些时候和2022年初完成。但如果罗沙司他的安全性问题是基于其作用机制,那么整个类别的药物都将处于危险之中。
早在2018年12月,罗沙司他(爱瑞卓)就获得了中国国家药品监督管理局(NMPA)的批准,用于治疗透析依赖的慢性肾病患者的贫血。2021年上半年,罗沙司他在中国的销售额达到9630万美元,已经实现盈利。今天, 在FDA挖掘出新的安全性数据后,NMPA是否应该重新评估爱瑞卓在中国的销售?

Why did the FDA Refuse FibroGen’s Anemic Drug Roxadustat?
Recently, the US FDA completed reviewing roxadustat submitted by FibroGen and AstraZeneca for the treatment of anemia caused by chronic kidney disease. If approved, roxadustat would be first-in-class prolyl hydroxylase inhibitor. Unfortunately, on August 11, 2021, following its Advisory Committee’s recommendation, the FDA rejected roxadustat for safety concerns. Meanwhile, the FDA has demanded another clinical trial of roxadustat on safety before it will consider its approval. This is a worst-case scenario for the drug. FibroGen’s and AstraZeneca’s investors likely will not be willing to fund an additional expensive Phase III trial without revenue contributions from roxadustat in the U.S.
FibroGen’s already beleaguered stock price continued to slide as news emerged, and the stock was trading at less than $13, having been above $52 in February this year.
Ironically, roxadustat has been on the market in China (brand name 爱瑞卓) and Japan for the treatment of renal anemia since 2018.
1.Anemia and chronic kidney disease
Three types of blood cells exist in human body: red blood cells, white blood cells and platelets in addition to plasma which takes up 55% volume of the blood. Red blood cells take up approximately 45% volume of the blood. They transport oxygen from lungs to other body parts. White cells defend us against bacterial and viral invasions. Platelets (less than 1% in blood), the third type of blood cells, are sticky little cell fragments that are involved in helping the blood coagulate.
Anemia is a disorder of blood involving a decreased amount of red blood cells or hemoglobin in circulation. Containing an iron atom bound to a heme group, hemoglobin is a protein in red blood cells that carries oxygen to cells throughout the body.
Chronic kidney disease, cancer, chronic inflammatory disorders, nutritional, and genetic deficiency can cause anemia. Anemia is particularly prevalent in patients with chronic kidney disease, which may be caused by a myriad of conditions including diabetes, hypertension, obesity, smoking, etc. Chronic kidney disease affects 840 million patients worldwide and is generally progressive, characterized by gradual loss of kidney function that may eventually lead to kidney failure. The disease frequently causes significant fatigue, cognitive dysfunction and decreased quality of life, and is associated with increased risk of hospitalization, cardiovascular complications and death.
Old treatments of anemia of chronic kidney disease include blood transfusion, iron supplement and vitamins. But these treatments have limited efficacy. Today’s standard-of-care are erythropoietin (EPO) therapies Epogen and Procrit by Amgen and Johnson & Johnson, respectively. Epogen and Procrit are erythropoiesis-stimulating agents.
Prolyl hydroxylase (PHD) inhibitors, such as roxadustat, can stabilize hypoxia-inducible factor (HIF) and induce erythropoietin production under normal conditions.1 They represent a novel pharmacological treatment of anemia associated with chronic diseases.
2.Prolyl hydroxylase and its inhibitors
Hypoxia enhances red blood cell synthesis by stimulating erythropoietin production by causing induction of hypoxia-inducible factor. Hypoxia-inducible factor was first discovered in 1993.2 In 1999, von Hippel−Lindau tumor suppressor protein (pVHL) was confirmed to play a crucial role in HIF-1 oxygen-dependent proteolysis.3 HIF-1α is recognized by E3 ubiquitin ligase, which tags HIF-1α with a signal by ubiquitination to carry it to proteasomes for degradation. This process is the same as PROTACs using the ubiquitin-proteasome system (UPS).
On the other hand, prolyl hydroxylase domain enzyme inhibition can stabilize hypoxia-inducible factor. Hypoxia-inducible factor stabilization also decreases hepcidin, a hormone of hepatic origin, which regulates iron homeostasis. Many orally active prolyl hydroxylase inhibitors like roxadustat, daprodustat, desidustat, and vadadustat are in late phase clinical trials.
As a pioneer prolyl hydroxylase inhibitor, roxadustat demonstrates synergistic effects against renal anemia, including the promotion of erythropoietin production and improvement in iron metabolism.
FibroGen’s roxadustat reversibly binds to and potently inhibits prolyl hydroxylase enzymes, reducing HIF-α breakdown and promoting HIF transcriptional activity.4 It is an orally active drug thus is superior to injectable erythropoiesis-stimulating agents, which are also known to raise the risk of clotting events and so are not approved for non-dialysis dependent patients. Possible approval of the drug in the non-dialysis dependent group led to blockbuster sales predictions.
Roxadustat has already been approved for use in China, Japan, Chile, and South Korea for chronic kidney disease anemia in both dialysis dependent and non-dialysis dependent patients. In Japan and some other markets, it is sold by Astellas.5 It has also been recommended for approval in Europe.
During the FDA’s review process, the agency had no question on roxadustat’s efficacy, but it had concerns about its safety: mainly with relation to the risk of serious thromboembolic events. After digging into clinical trial data, the FDA reviewers linked roxadustat to increased risk of death, blood clots, serious infections and more compared with erythropoietin therapy in the dialysis-dependent population and with placebo in the non-dialysis-dependent group.
After the FDA’s internal review document revealed a slew of new safety problems previously unknown to investors, the Advisory Committee voted 13-1 against roxadustat’s approval in non-dialysis patients and 12-2 against for dialysis-dependent patients during a July meeting. FibroGen scientists “directly responsible” for the safety data error were promptly fired.
The question on everyone’s mind is: Is roxadustat’s safety issue molecule-based? Or is it mechanism-based? I would hope that it is the former.
If it were the former, roxadustat’s delay provides an opportunity for a rival drug in the oral prolyl hydroxylase inhibitor class, GlaxoSmithKline’s daprodustat, to catch up with roxadustat in the US market. Phase III readouts for daprodustat are due later this year and in early 2022. But if roxadustat’s safety issue were mechanism-based, the whole class of drugs would be in danger.
Back in December 2018, roxadustat was approved by the National Medical Products Administration (NMPA) for the treatment of anemia in China for patients with chronic kidney disease who were dialysis dependent. In China, roxadustat sales reached $96.3 million in the first half of 2021 and the business has become profitable. With newly surfaced safety data dug out by the FDA, should NMPA re-evaluate 爱瑞卓’s safety profile?
1.Joharapurkar, A. A.; Pandya, V. B.; Patel, V. J.; Desai, R. C.; Jain, M. R. Prolyl Hydroxylase Inhibitors: A Breakthrough in the Therapy of Anemia Associated with Chronic Diseases. J. Med. Chem. 2018, 61, 6964–6982.
2.Wang, G. L.; Semenza, G. L. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 1993, 268, 21513–21518.
3.Maxwell, P. H.; Wiesener, M. S.; Chang, G. W.; Clifford, S. C.; Vaud, E. C.; Cockman, M. E.; Wykoff, C. C.; Pugh, C. W.; Maher, E. R.; Ratcliffe, P. J. The tumour suppressor protein VHL targets hypoxia--inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275.
4.Su, K.; Li, Z.; Yu, Y.; Zhang, X. The prolyl hydroxylase inhibitor roxadustat: Paradigm in drug discovery and prospects for clinical application beyond anemia. Drug Discovery Today 2020, 25, 1262–1269.
5.Dhillon, S. Roxadustat: First Global Approval. Drugs 2019, 79, 563–572.














 个人中心
个人中心
 我是园区
我是园区




