
致力于抗肿瘤新药开发的中美跨国制药公司徐诺药业在临床前研究中发现其候选新药艾贝司他可以高效穿过血脑屏障,此项研究与美国内布拉斯加大学医学中心合作完成。
研究发现:
· 艾贝司他可高效地穿过血脑屏障,并与替莫唑胺产生明显的协同效应;
· 在实验动物模型中,艾贝司他与替莫唑胺联用的抗肿瘤作用明显优于替莫唑胺单药或替莫唑胺与伏立诺他联用。
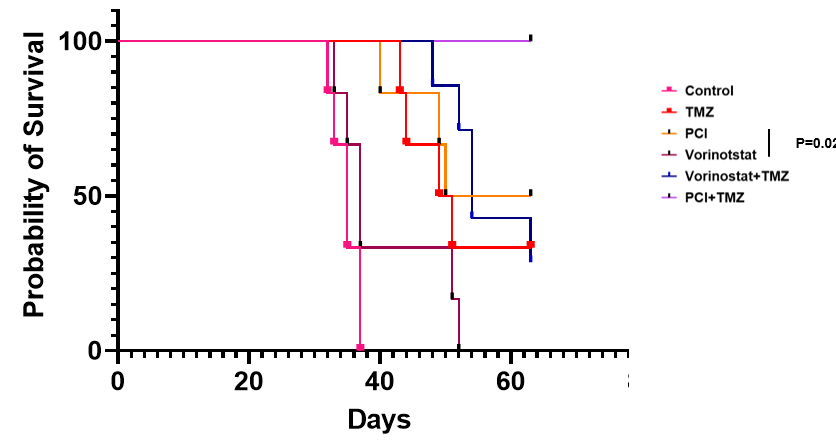
鉴于以上优良的临床前数据,徐诺药业计划与内布拉斯加大学医学中心联合开展一项治疗胶质瘤的1期临床试验,探索艾贝司他与替莫唑胺/放疗联用治疗胶质瘤的安全性和有效性。同时,此项研究已向 美国国立卫生研究院(NIH)申请研究经费。
Title: A Phase I Study of Metronomic Temozolomide with PCI-24781 for Patients with Recurrent High Grade Glioma
题目:替莫唑胺化疗与艾贝司他联用治疗复发性高级别胶质瘤的I期临床研究
Objectives: To assess the dose limiting toxicity and efficacy of metronomic temozolomide and PCI-24781 for recurrent high grade glioma patients who have received prior radiation therapy and standard temozolomide.
目的:评估替莫唑胺化疗和艾贝司他联用对既往放射治疗和替莫唑胺标准治疗后复发的高级别胶质瘤患者的剂量限制性毒性和有效性。
脑瘤的发病率仅次于胃、子宫、乳腺及食管肿瘤的发病率,约占全身肿瘤的2%。由于绝大多数抗肿瘤药物(包括PD-1和PD-L1类药)无法有效穿过血脑屏障,治疗脑瘤新药一直没有突破。替莫唑胺和艾贝司他联用有望为脑瘤患者带来新的治疗手段。

关于徐诺药业
徐诺药业是一家专注于肿瘤药物开发的生物制药公司。
徐诺药业目前的产品管线主要包括三款候选药物,艾贝司他、 XP-105 和 XP-102。公司拥有这些药物的全球独家开发、生产和商业化权益。其领先的候选药物艾贝司他正在进行治疗肾细胞癌(与培唑帕尼联用)和单药治疗非霍奇金淋巴瘤的全球关键临床试验。徐诺药业另外一款临床阶段候选药物为可进入临床2期的XP-105(BI 860585),这是一个ATP竞争性的mTORC1 / 2抑制剂,对多种实体瘤有效。徐诺药业的临床前候选药物XP-102(BI 882370)是一个泛-RAF抑制剂。
投资者关系、媒体和商务拓展请联系:
angela.feng@xynomicpharma.com
Use of
Forward-Looking Statements
This press release contains “forward-looking” statements made pursuant to the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. For this purpose, any statements contained herein that are not statements of historical fact may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “should,” “expects,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential,” or “continue,” or the negative of these terms or other similar expressions. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
Xynomic has based these forward-looking statements largely on its current expectations and projections about future events and trends that it believes may affect its business, financial condition and results of operations. These forward-looking statements speak only as of the date of this press release and are subject to a number of risks, uncertainties and assumptions including, without limitation, risks related Xynomic’s financial position and need for additional capital to complete the planned trials and support its continuing operation, risks related to uncertainty in maintaining and obtaining regulatory approval and ultimately commercialize its drug candidates or delays in doing so; and the risks more fully described in Xynomic’s filings that Xynomic may make with the SEC in the future. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future.



 个人中心
个人中心
 我是园区
我是园区




